Basics of Gas Chromatography
3-3 Splitless Injection Method
The splitless injection method is primarily used in microanalysis. The split vent is closed during sample injection and opens when the sample is almost fully introduced to the column. At this time, residual material in the liner is expelled. As the injection volume is large, the sample tends to spread along the column. Therefore, the sample must be efficiently introduced to the column and spreading must be mitigated through the concentration effect (discussed below).
<Inner diameter of liner>
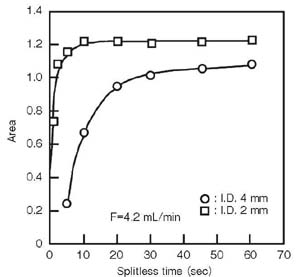
The sample injected into the liner must be efficiently introduced to the column. For this purpose, a liner with a narrow inner diameter is desired. The linear velocity in the liner is quickly increased and introduced to the column. A liner with a narrow I.D. quickly feeds the sample into the column, but if a large sample volume is injected into the liner, it may overflow via evaporation and expansion when passing into the column.
<Carrier gas flow rate>
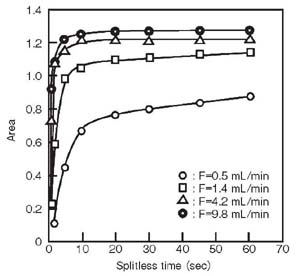
The carrier gas flow velocity is the flow velocity in the liner. Therefore increasing the carrier gas rate is one of the ways to avoid sample overflow in the liner.
<Column temperature>
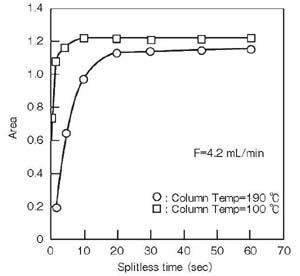
If the column temperature exceeds the inlet temperature, the solvent may expand (vaporize in extreme cases) at the column inlet and interfere with sample introduction to the column.
3-3-1 Sample Concentration at the Column Inlet
Once the sample has been efficiently introduced to the column, its concentration at the column inlet becomes important. If the sample is inadequately enriched at the column inlet, the sensitivity of the column is compromised.
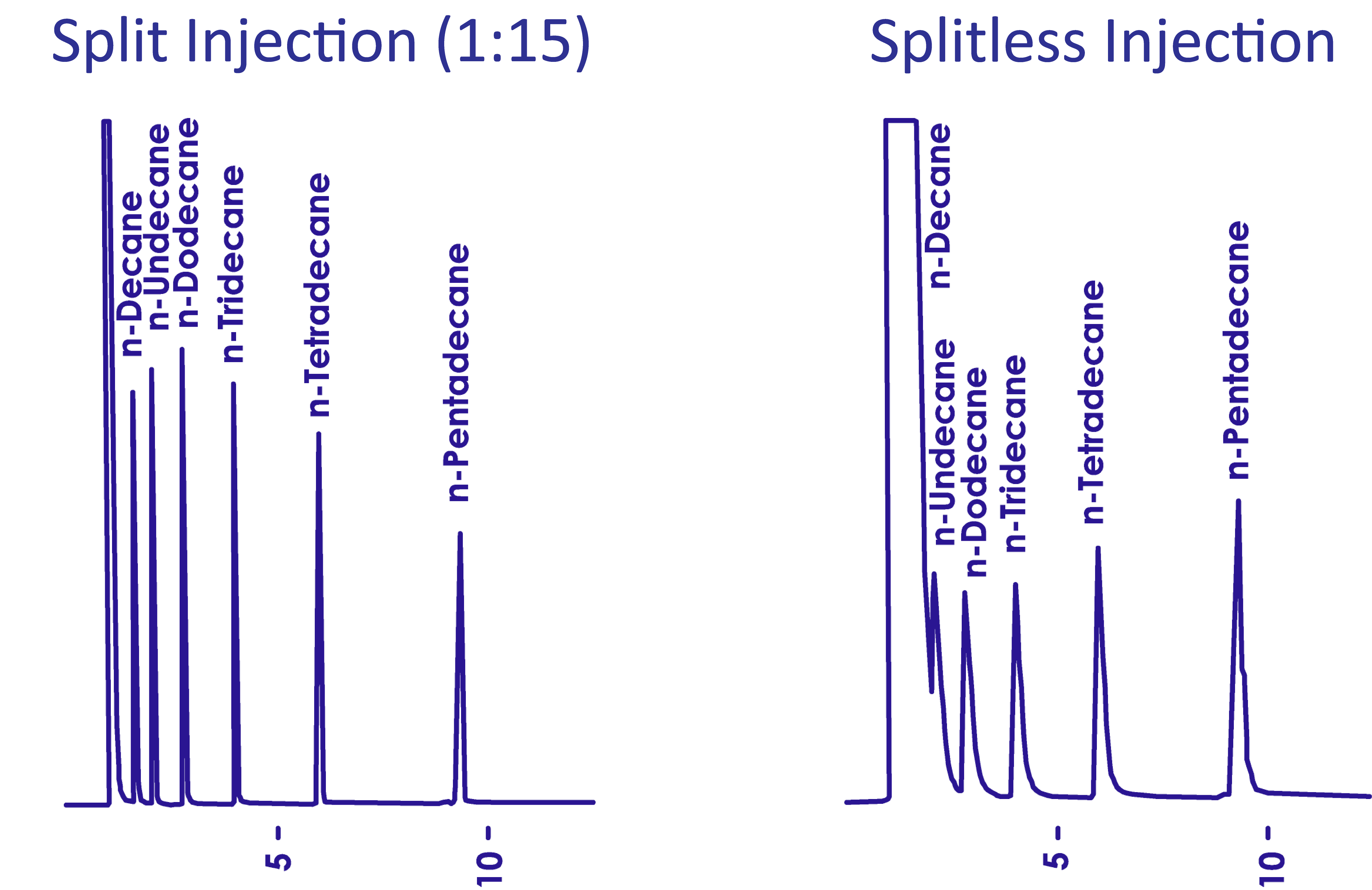
The chromatograms above show the same sample analyzed under the same conditions but introduced by different injection methods: the split injection method (split ratio 1:15) and the splitless injection method. Although splitless injection introduced nearly 15 times more sample than split injection, it achieved lower sensitivity because the concentration effect was not established. In successful analyses, the sample at the column inlet is concentrated via the solvent effect, retention gap effect, and cold trapping. These effects are discussed below.
The Solvent Effect
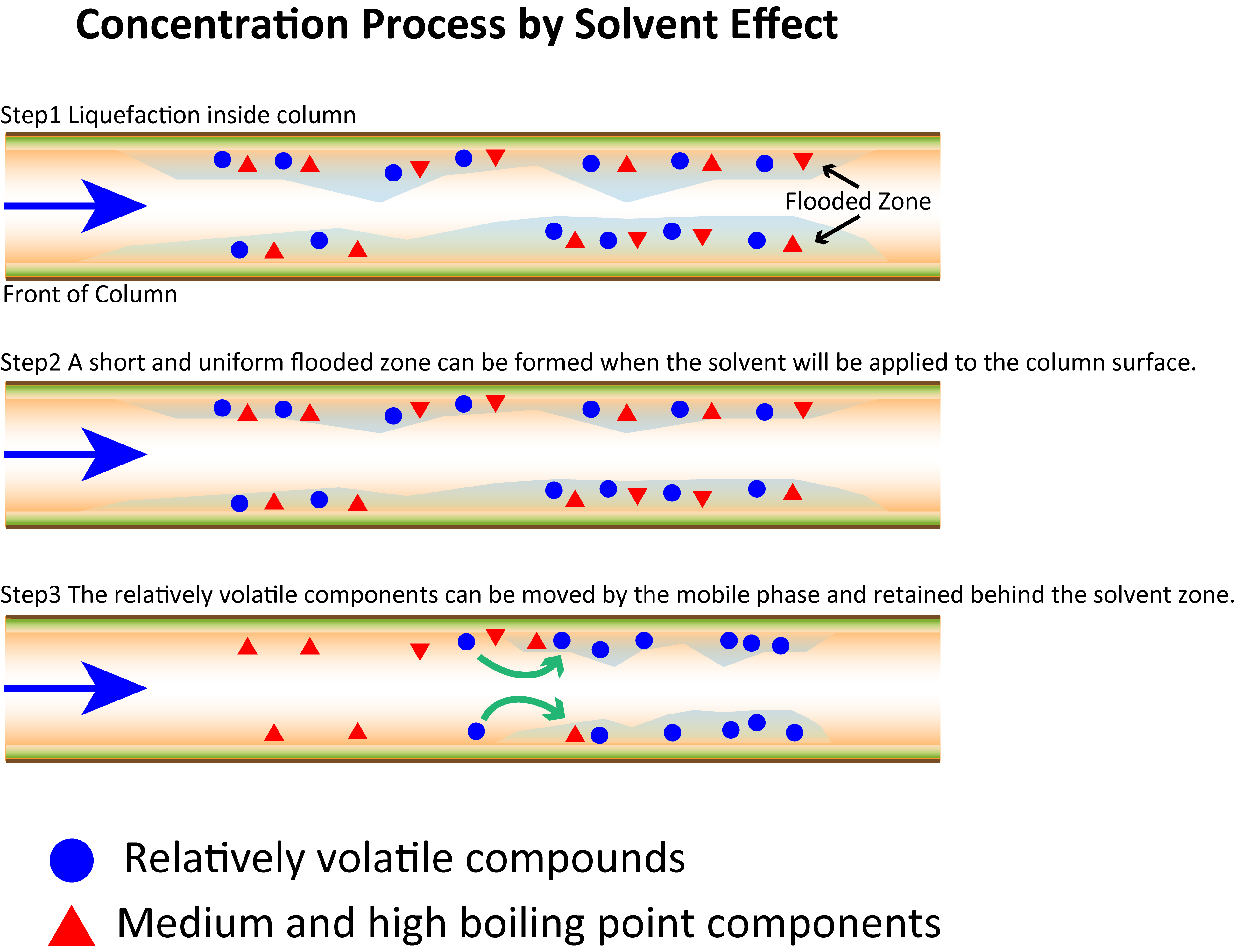
The solvent effect occurs when a large amount of solvent enters a column whose temperature is below the boiling point of the solvent. The vaporized solvent at the inlet returns to liquid in the column, creating a flooded zone into which the analytes flow. The solvent gradually evaporates from the column inlet side. After vaporization of the solvent, the volatile components of the analytes left on the column surface are vaporized and transported by the mobile phase. Meanwhile, the volatile components of the analytes are collected and concentrated in the solvent, which transfers much more slowly than the mobile phase and hence remains on the column surface (. As the medium- and high-boiling components do not vaporize, they spread over the length of the flood zone, remain in the column, and are transported through the column as the temperature rises.
To ensure an effective solvent effect, the column temperature must be below the boiling point of the solvent. The chromatograms below are the result of split injection into a 40°C column with different dilutions of nitrogen gas, iso-pentane, and n-hexane.
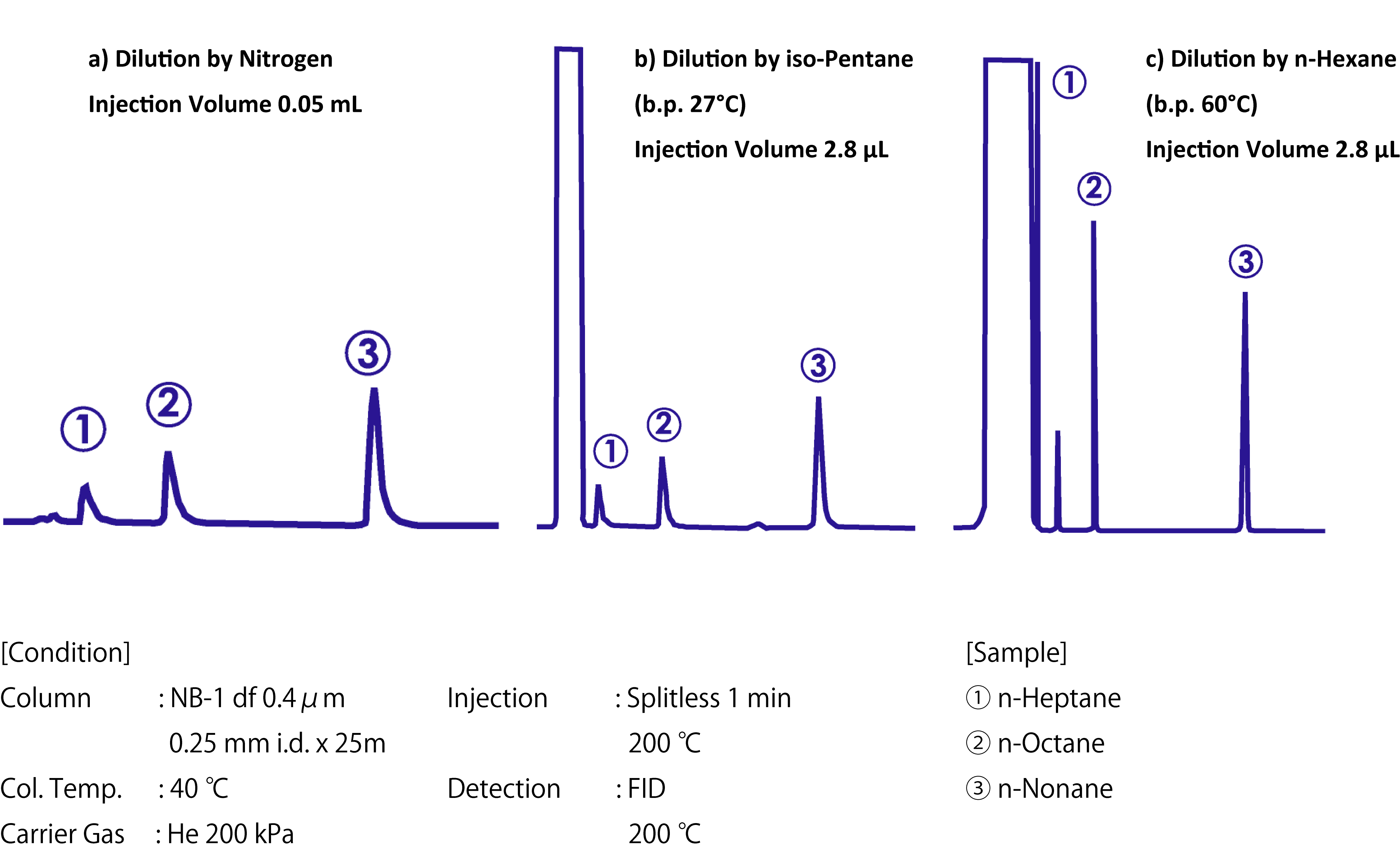
The solvent effect widened the peaks of the compounds in all diluents except n-hexane. Thus, the column temperature should usually be set to 10°C–40ºC below the boiling point of the solvent used. The low boiling–point components failed to concentrate so their peaks were also broadened.
The Retention Gap Effect
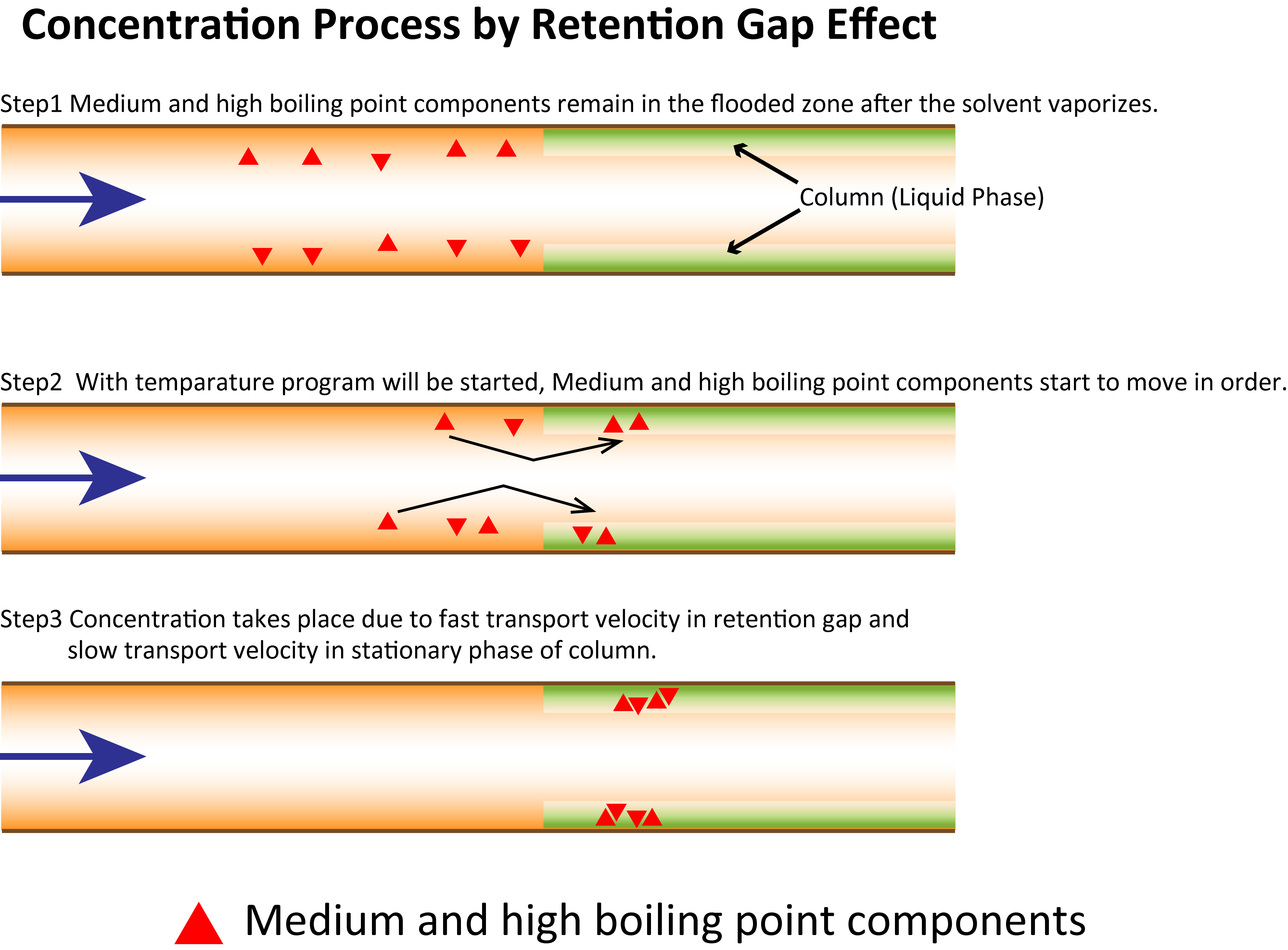
If the injection volume exceeds 1 µL or the solvent poorly blends with the liquid phase, the flood zone will widen at the column inlet. In such cases, a column coated with a thin liquid phase or a capillary tube with no liquid phase can be installed before the analytical column. Such a column is called a retention gap. Medium and high boiling–point components are spread within the retention gap and begin migrating as the temperature rises. Eventually they accumulate at the tip of the analytical column. When connected, the retention gap exerts two major effects. First, it improves the compatibility of the liquid phase with the solvent, thereby reducing the spread of the flooded zone. Second, it weakly retains and quickly desorbs the analytes, accelerating their progress into the analytical column and increasing the enrichment efficiency.
The Cold Trap Effect
If the column temperature is much lower than the boiling point of the analyte at the time of sample injection, the analyte will migrate slowly through the column and becomes concentrated at the column tip. This phenomenon, called the cold trap effect, occurs when the vaporized sample enters a column that is at least 100°C colder than the boiling point of the component to be analyzed.
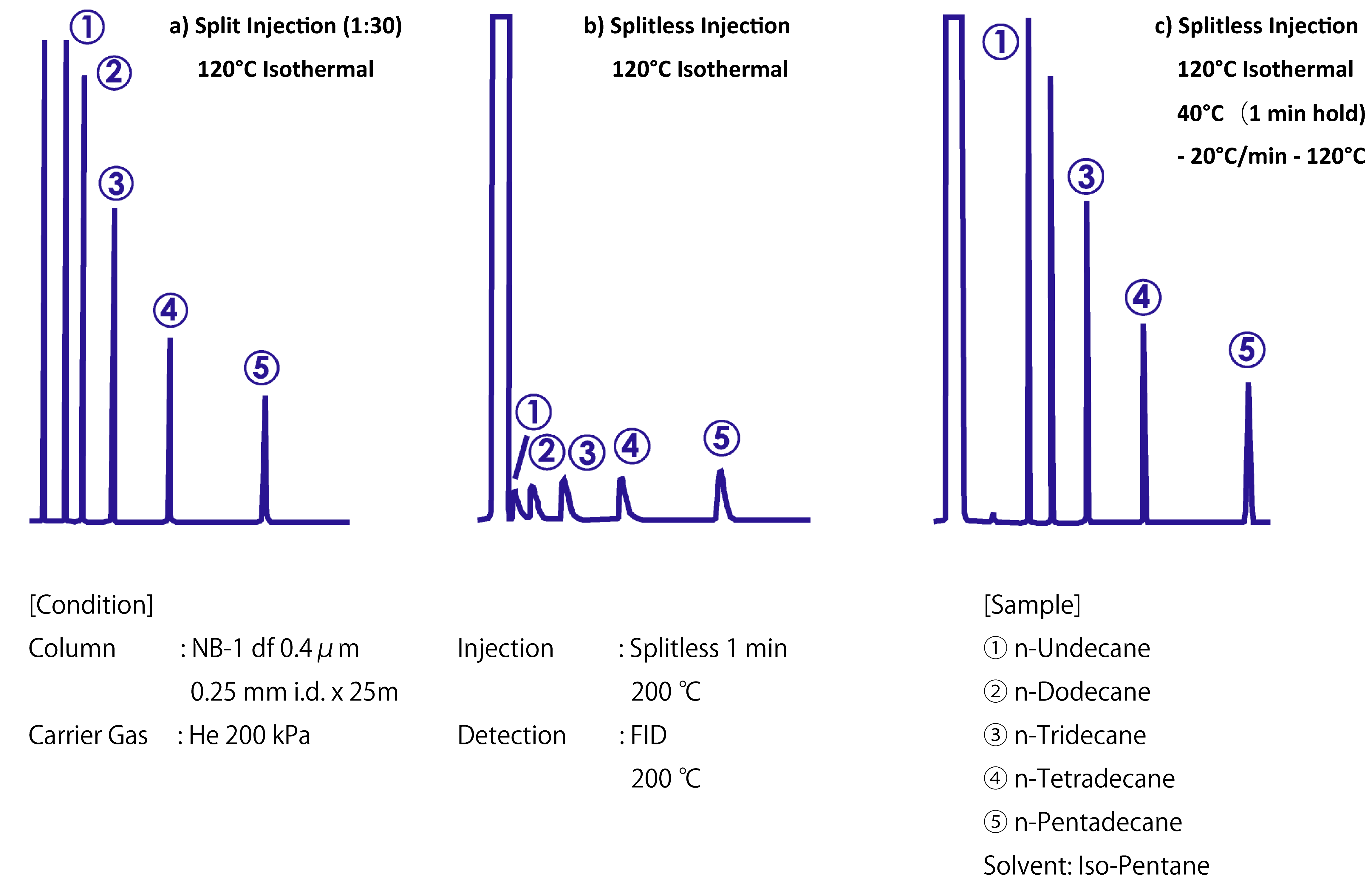
To avoid the abovementioned solvent effect, the dilution solvent was iso-pentane. The peak width was clearly wider in the splitless injection case (b) than in the split injection case (a). However, when the sample entered a cold column (case (c)), sharp peaks were obtained even after splitless injection because the low column temperature elicited the cold trap effect.


























