Even for the same types of HPLC columns, their separation performance and the robustness of the method depend a lot on how to use them Here, we present some tips to obtain better results with chemically bonded silica gel columns, which are the most widely used columns today.
1. How to Obtain Good Peak Shapes
In general, sharp peaks are preferred in chromatography because broad peaks can easily overlap each other In addition, since the peak area is proportional to the concentration, the narrower the peak is, the higher the peak height becomes This is favored in terms of sensitivity.
1-1. Minimizing Sample A dsorption
When analytes are adsorbed onto the packing material, the peak shapes become bad and most of them are asymmetric If such a phenomenon occurs, it is necessary to take measures such as using a well end capped column.
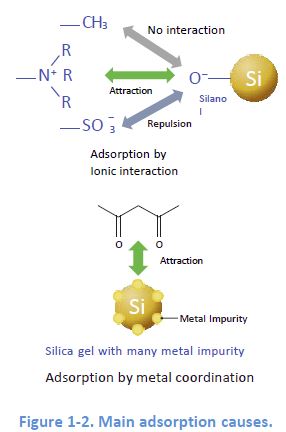
Representative Cause 1: Adsorption by Electrostatic I nteraction
When the ionic functional groups, for example, silanol groups SiO remain on the packing material, electrostatic interaction can happen If this happens in reversed phase mode, it can cause peak distortions.
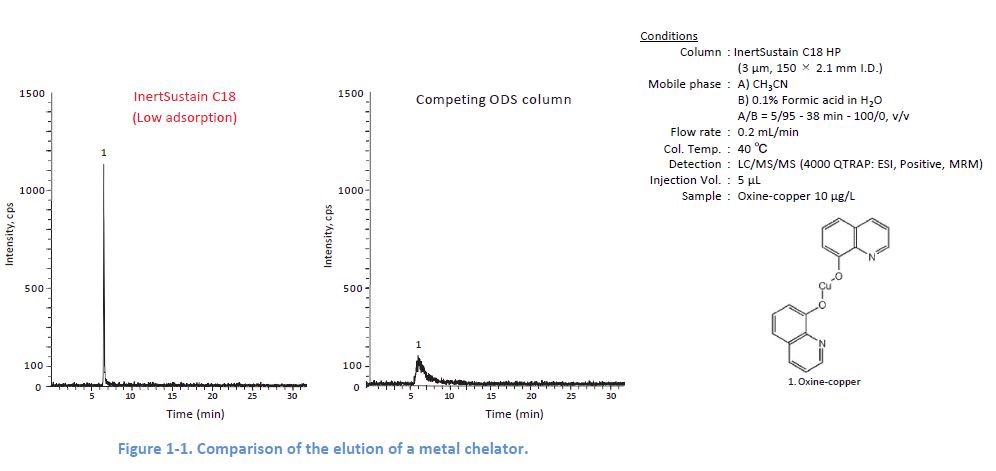
Representative Cause 2: Adsorption by Metal Coordination
The inclusion of metals in packing silica can lead to adsorption of metal chelators contained in a sample This adsorption sometimes causes no elution of the target compounds.
1-2. Choosing Small Particles
Even for the same packing material, smaller particles yield better column efficiency However, note that smaller particles generate higher column pressure Please check the pressure resistance of your system.
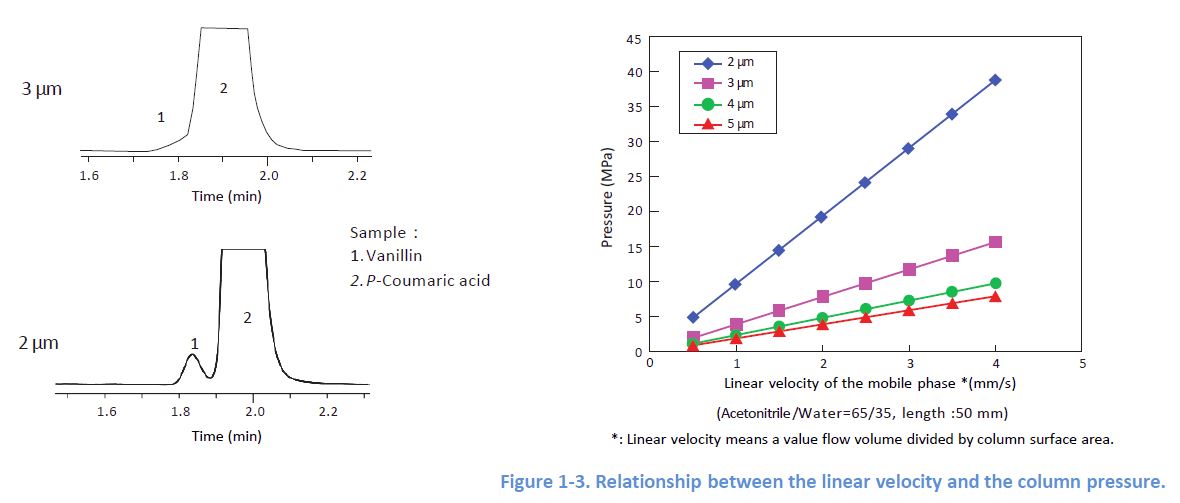
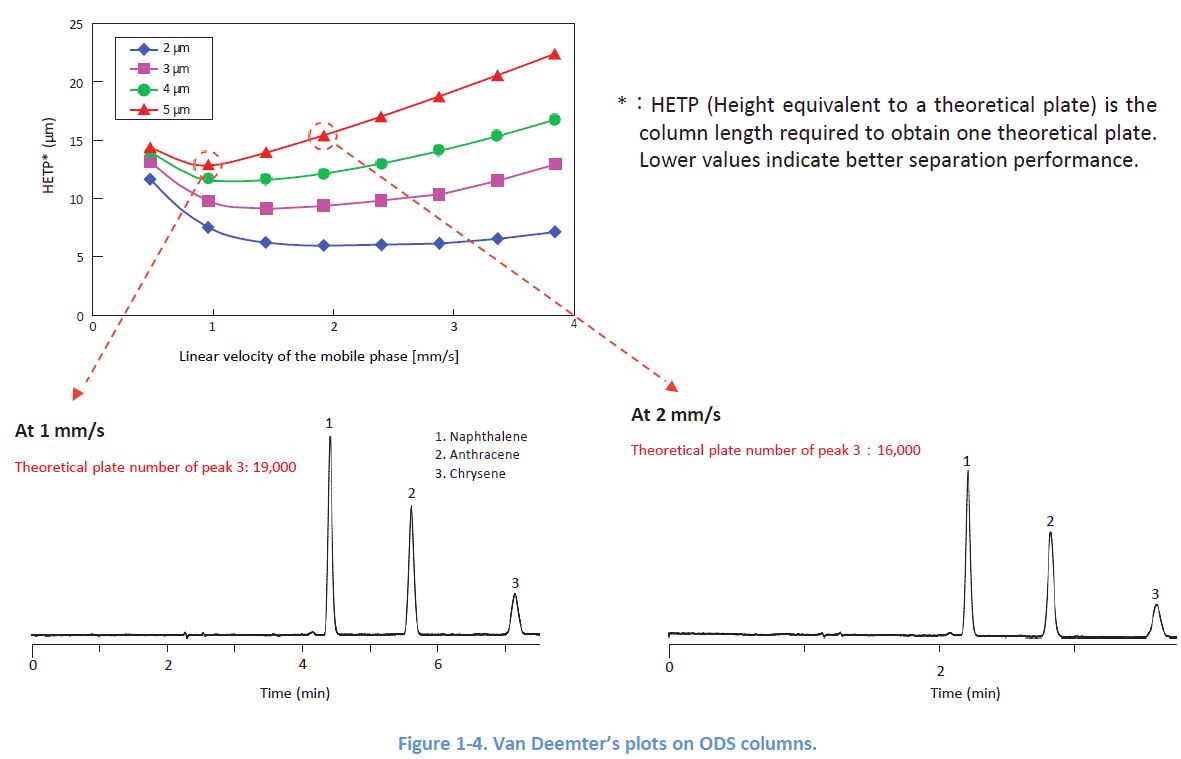
1-3. Effect of the Flow Rate
Peak widths depend on the linear velocity of the mobile phase, and each column has its optimal In order to obtain sharp peaks, it is desirable to apply a flow rate which delivers a linear velocity close to the optimal.
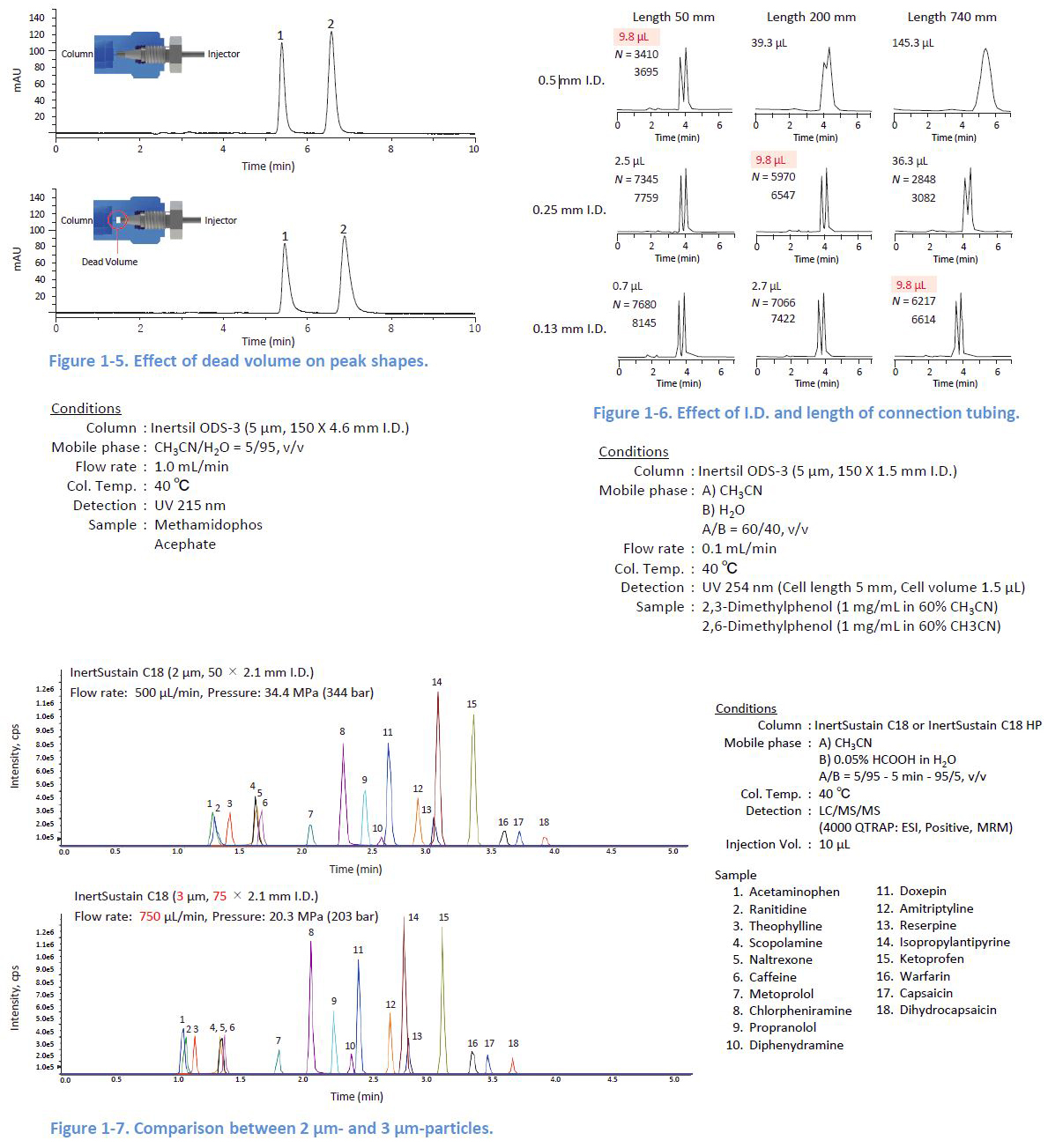
1-4. Minimizing the Dead Volume
If there is a dead volume between the tubing and the column, peaks are distorted This also happens when the relative volume of the tubing to the column is large Columns with a small internal diameter (I D or a short length are easily affected To reduce the effect of the dead volume, using long columns is one solution Figure 1 7 shows a comparison between a 50 mm long column packed with 2 µm particles and a 75 mm long column packed with 3 µm particles With the longer column length, even larger particles provide better peaks because the effect of the dead volume is less significant.
1-5. Pore size of silica particles
For compounds which have a molecular weight larger than 5 000 it is sometimes necessary to use particles having wide pores to obtain good peak shapes For example, Inertsil WP 300 C 18 is a good option for these compounds.
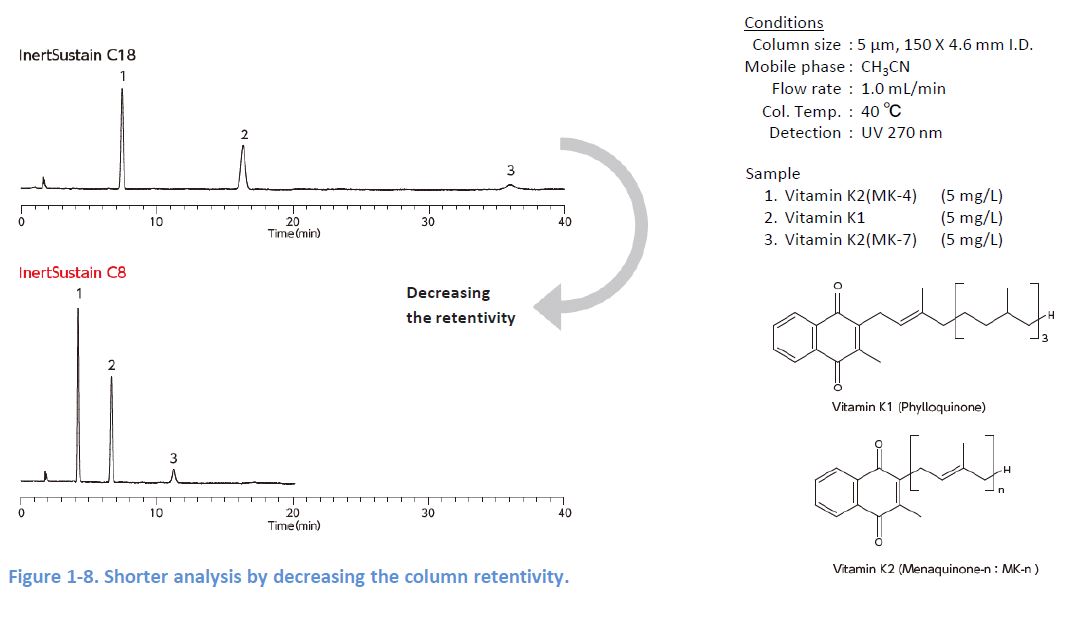
1-6. Fast Elution
In general analytes spread more as it takes longer from injection to detection, and this results in broader peaks Therefore, sensitivity can be improved by adopting analytical conditions which enable short analysis 1 increasing the column retentivity 2 shorten the column length, and 3 changing the mobile phase composition However, note that these methods might worsen your separation.
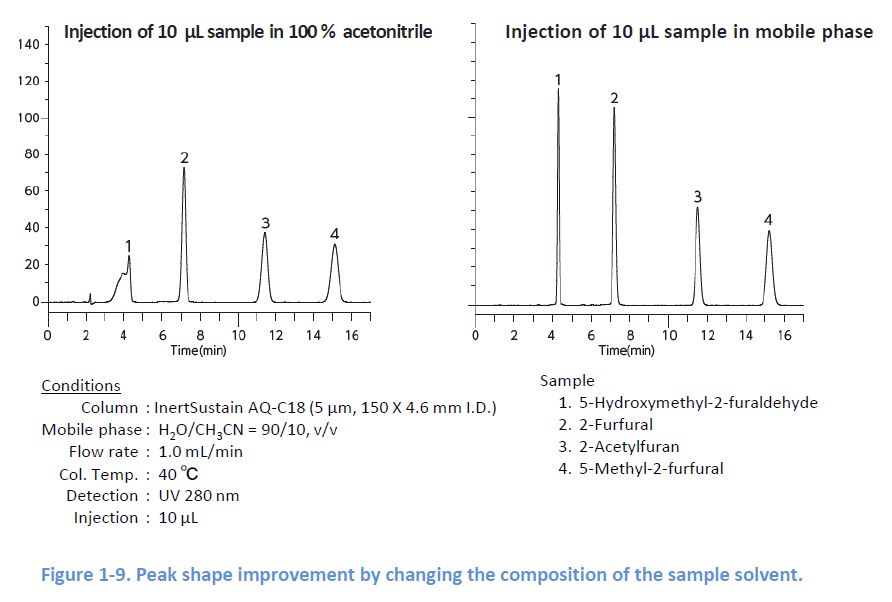
1-7. Composition of the Sample Solvent
If there is a mismatch between the sample solvent and the mobile phase, peak shapes can be distorted because the sample solvent works as the mobile phase soon after the injection This has significant influence on the peak shapes when the sample solvent has higher elution strength In this case, changing the composition of the sample solvent or the injection volume can improve the peak shapes.