1. Sample Solution
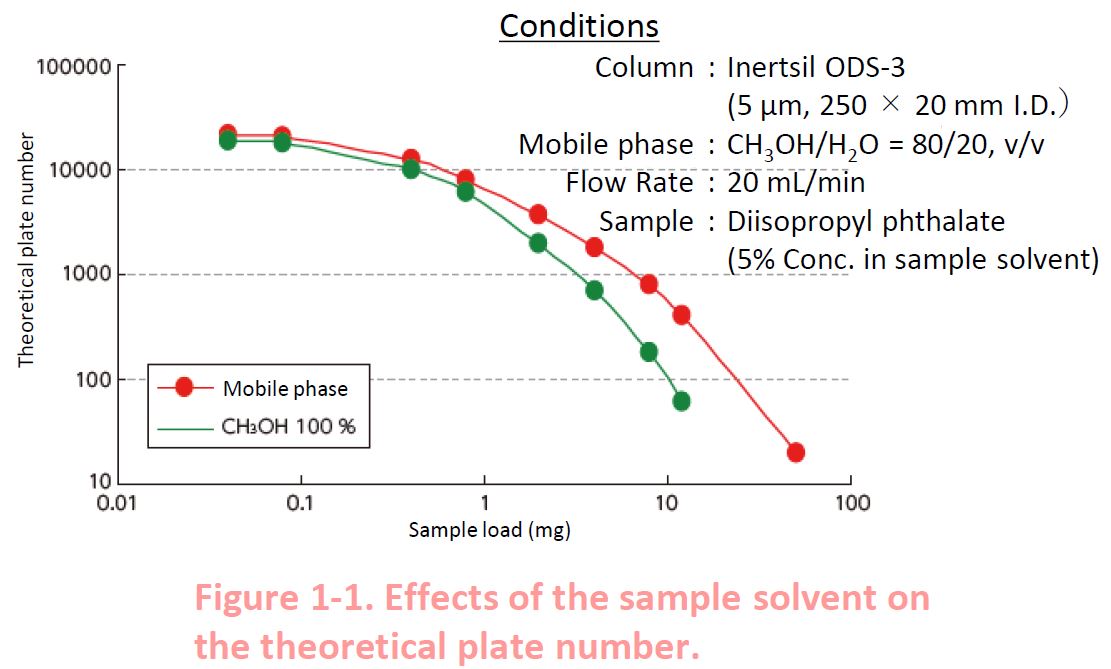
1-1. Sample Solvent
Please note the effects of the sample solvent Figure 1 1 shows that the sample solvent of 100 methanol distorts the peak shape and lowers the theoretical plate numbers compared to the mobile phase (methanol/water 80 20 v/v) as the sample solvent This is because the solubility of the analyte in methanol is higher than in the mobile phase This effect is notable in preparative HPLC The details of this effect in isocratic mode and gradient mode are described below.
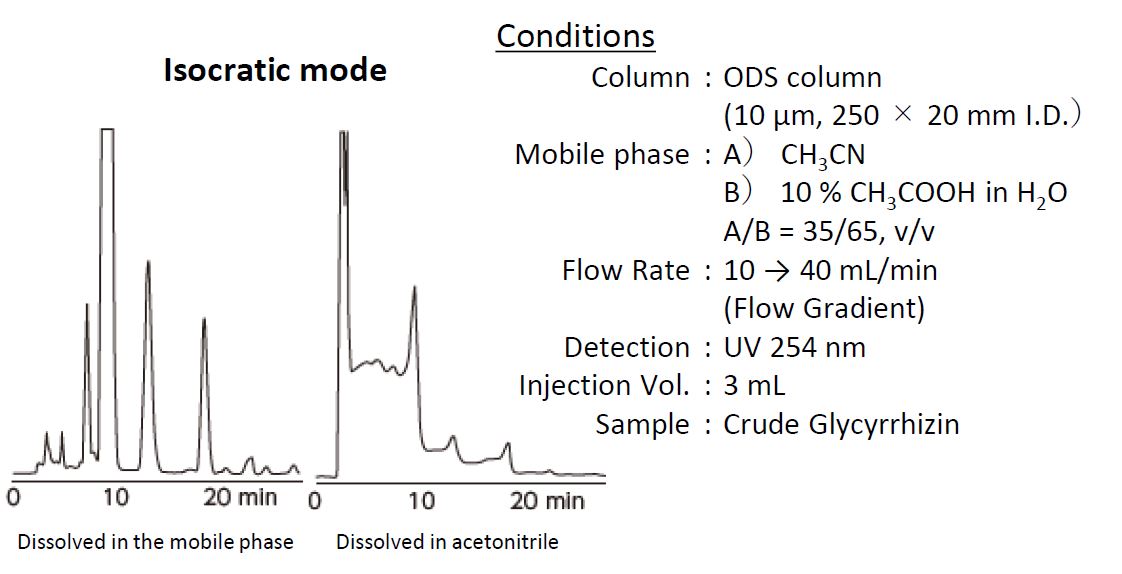
1-2. Isocratic Elution
When the flow rate is set to 10 mL/min and 3 mL of sample dissolved in 100 acetonitrile is injected, nearly 100 acetonitrile flows in the column for 18 s This suppresses the interaction between the analyte and the stationary phase because the elution strength of 100 acetonitrile is stronger than the mobile phase 35 acetonitrile).
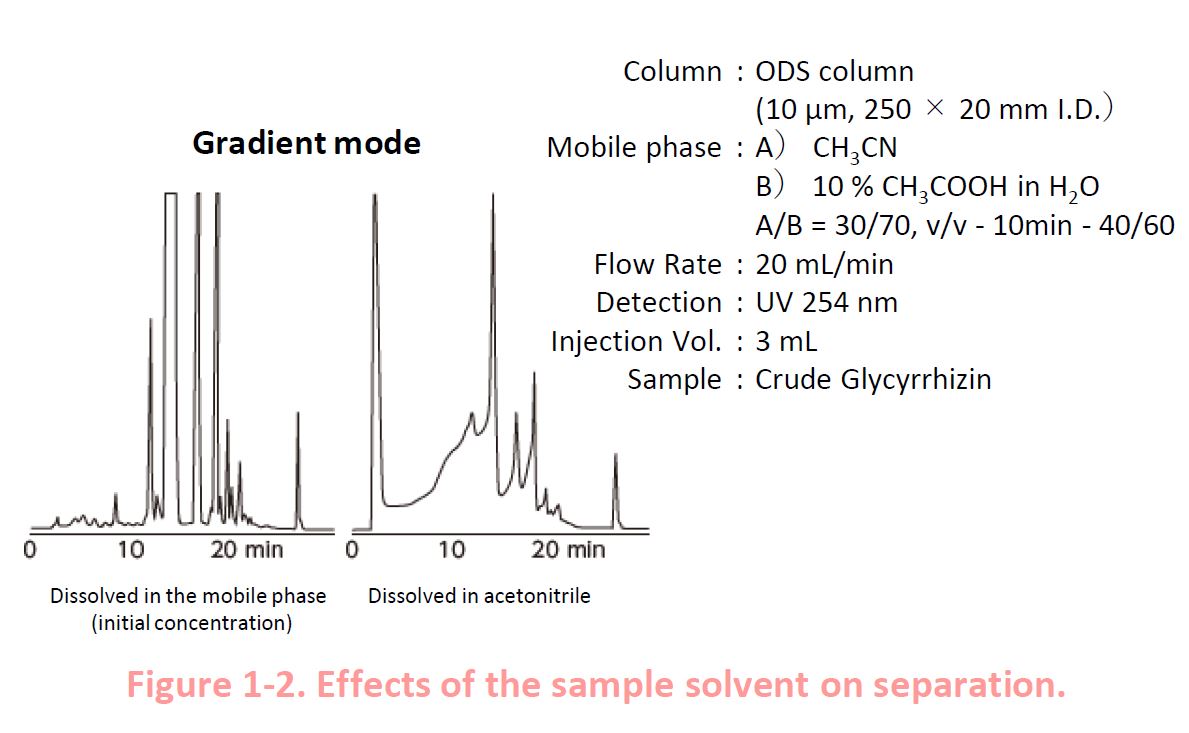
1-3. Gradient Elution
In gradient elution, analytes are concentrated at the head of the column, and elute them by gradually increasing the content of the stronger solvent in the mobile phase In the example shown on the right, the acetonitrile content in the mobile phase is gradually increased Peak distortion is observed also in this case.
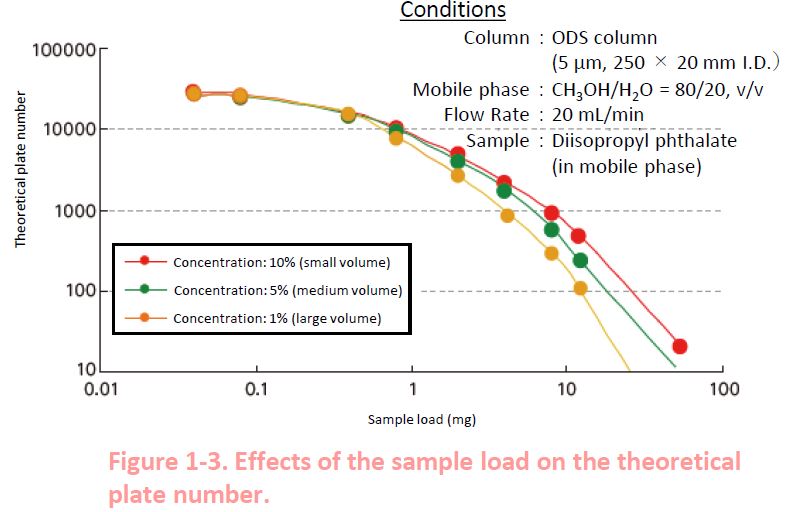
1-4. Sample Concentration
For a given amount of sample, it is better to inject a small volume of high concentration sample than a large volume of low concentration sample in terms of the theoretical plate number.*
Injection of 1 mL of 1% sample (sample load = 10 mg)
Injection of 0.1 mL of 10% sample (sample load = 10 mg)
*Some polymer supports have limits in sample load and concentration.