Basics of Gas Chromatography
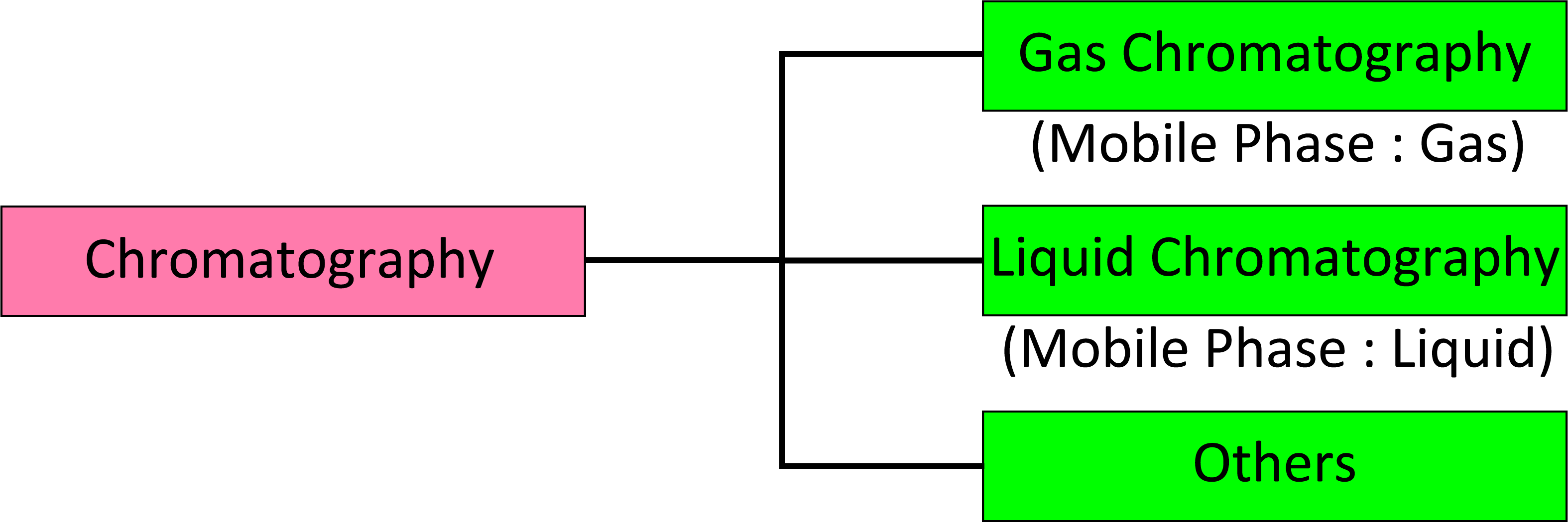
1-1 Classification of Chromatography
Chromatography performed in the equilibrium state separates the components of a sample in mobile phase through a stationary phase. The process relies on the different magnitudes of interaction between each component of the sample and both phases. Gas chromatography (GC) uses gas as the mobile phase, whereas liquid chromatography uses liquid as the mobile phase. To be eligible for analysis by GC, the sample components must be able to move in the mobile gas phase. A mobile object in a gas is either the gas itself or a compound that becomes gaseous at sufficiently high temperature. More precisely, this mobile object has a vapor pressure of approximately 10-mmHg or higher at the column's operating temperature.
Gas Chromatography (GC)
In GC, the sample is poured into the column together with the mobile (gas) phase and its components are separated by the interaction (adsorption and distribution) between the sample and the stationary phase. If the sample is analyzable by GC, its components will move in the gas of the mobile phase. A mobile object in a gas is either the gas itself or something that can vaporize at sufficiently high temperature.
High performance liquid chromatography (HPLC)
In HPLC, the sample is poured into the column together with the mobile (liquid) phase and its components are separated by the interaction (via mechanisms such as adsorption, distribution, ion exchange, and size exclusion) of the sample with the stationary phase.


























