GC Basic Knowledge Table of Contents
2-3 Other Factors Affecting the Analysis
Factors other than columns can affect a chromatographic analysis. These factors are discussed below.
2-3-1 Types of Carrier Gases
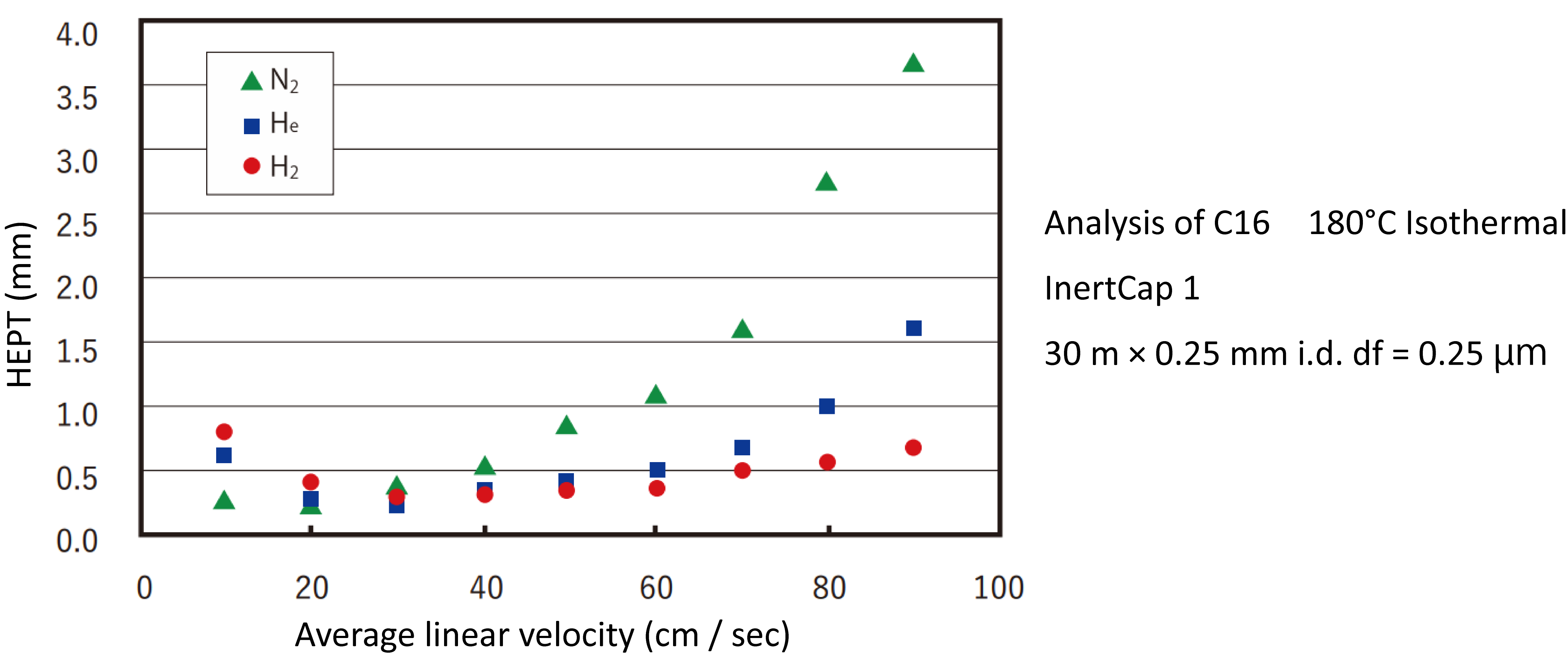
Carrier gases used in capillary columns include nitrogen, hydrogen, and helium. Nitrogen is inexpensive and safe but is disadvantaged by slow optimum linear velocity, which lengthens the analysis time, and a narrow range of optimum flow velocities. Hydrogen is an ideal carrier gas because it is inexpensive and has a wide range of optimum flow velocities but is beset with safety issues. The typical carrier has is helium, which is expensive but safe and has an acceptably wide optimum velocity range. These advantages and drawbacks are summarized below:
- Nitrogen: Inexpensive and safe, but its slow optimum linear velocity extends the analysis time. The optimum flow range is narrow.
- Hydrogen: Inexpensive and has a wide optimum flow range, but raises safety concerns
- Helium: expensive, but has a wide optimum velocity range and high stability
2-3-2 Velocity of the Carrier Gas
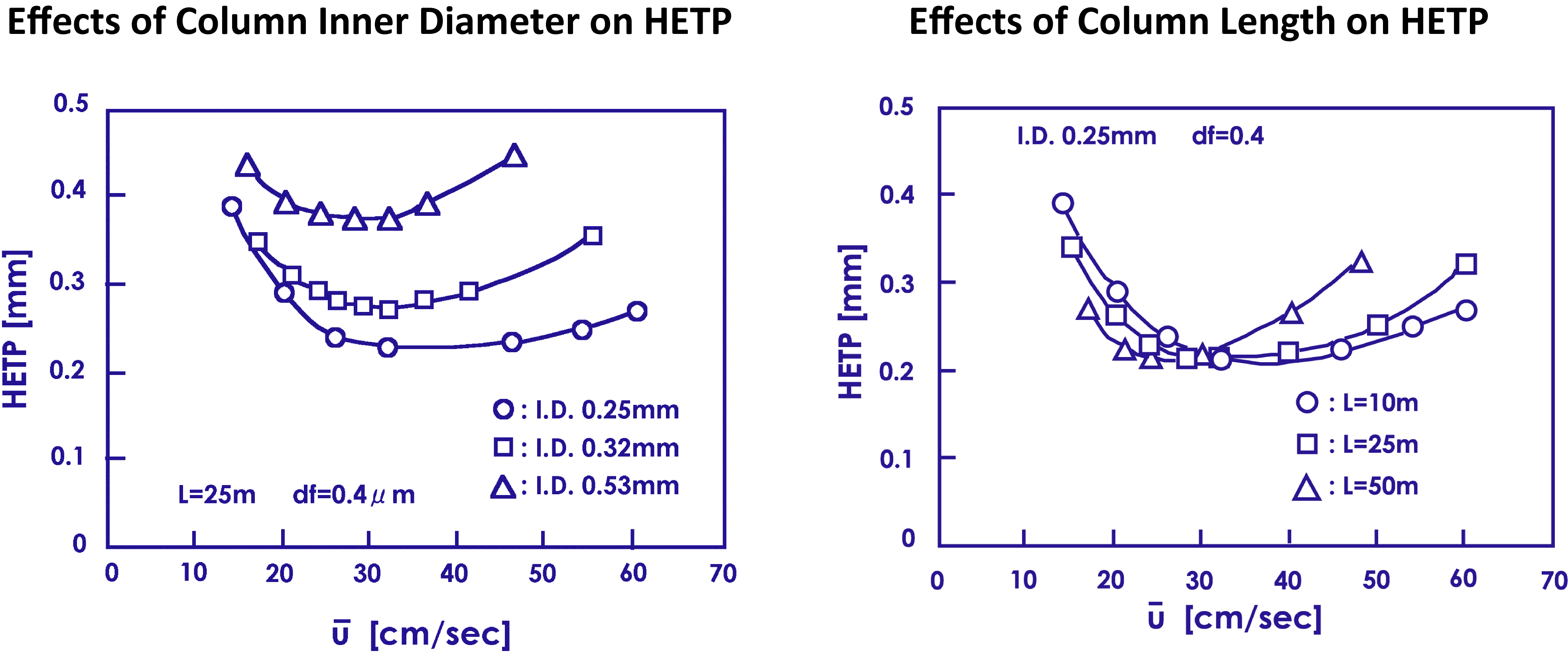
The carrier gas velocity is an important factor in capillary column analysis. Increasing the flow rate reduces the analysis time but the flow rate must be optimally selected for good separation. The optimum flow rate depends on the type of carrier gas, column inner diameter, and column length. The dependences of optimum flow rate on the inner diameter and length of a column when helium is used as the carrier gas. As the inner diameter reduces, the HETP becomes lower over a wider range. Meanwhile, shortening the column length widens the range of optimum flow velocities.
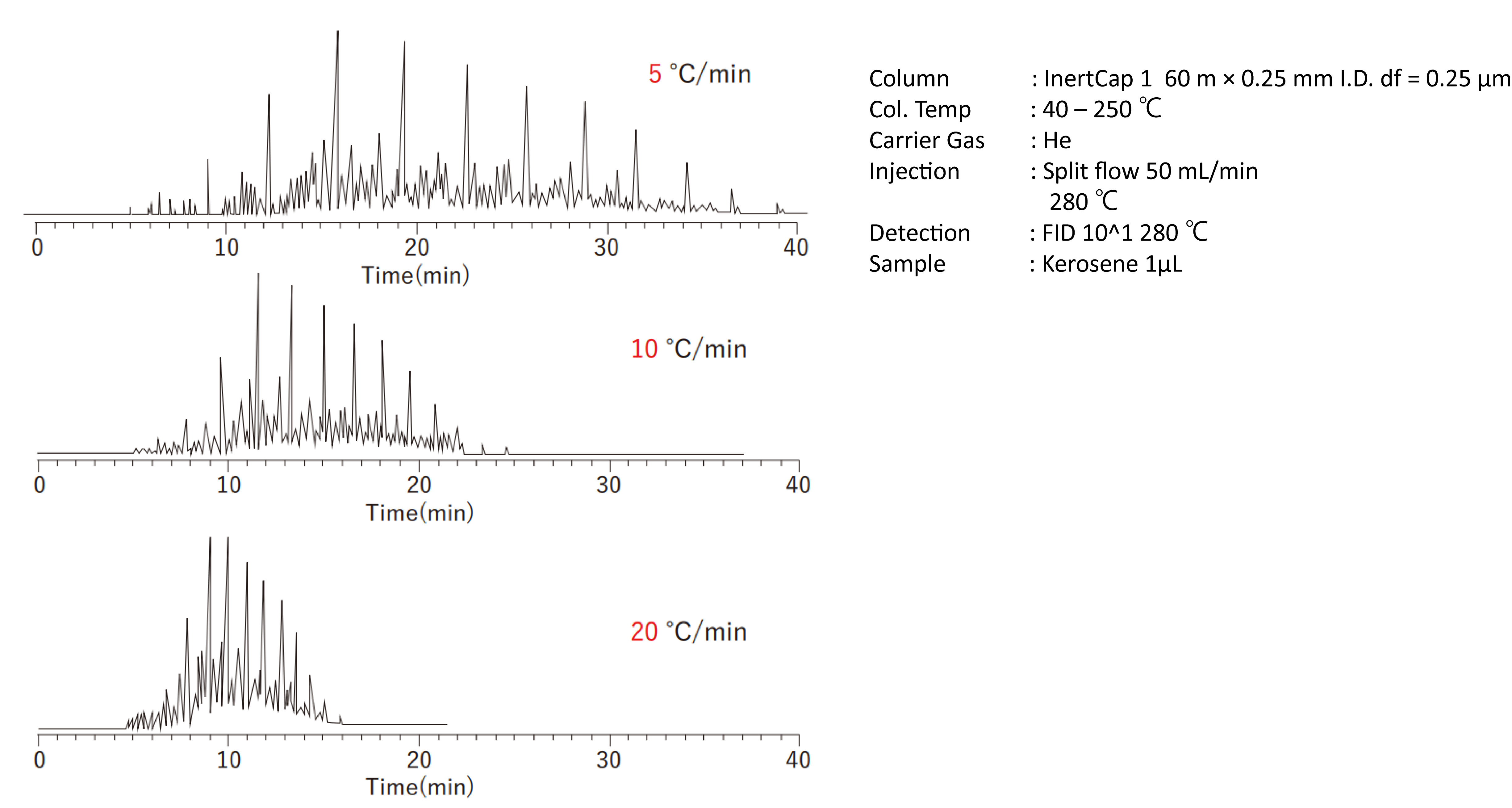
2-3-3 Column Temperature
Increasing the column temperature lowers the retention ratio and shortens the analysis time. The column temperature can be set by two methods: the isothermal method and the programmed temperature method. The isothermal method is used when the number of components is relatively small. As the analysis is performed at constant temperature, the next sample can be introduced immediately after the previous analysis is completed. This method also avoids rising baseline due to bleed. However, the isothermal method cannot easily perform simultaneous analyses of components with low and high-boiling points because the elution temperatures of the components are very different. The programmed temperature method, in which the temperature is raised from low to high, can yield clear peaks of components with low to high-boiling points. Note that the programmed temperature method will not change the elution order of compounds in the same family sequence, but may change the elution order in a multicomponent mixed sample.