Basics of Gas Chromatography
1-3 Column Principles
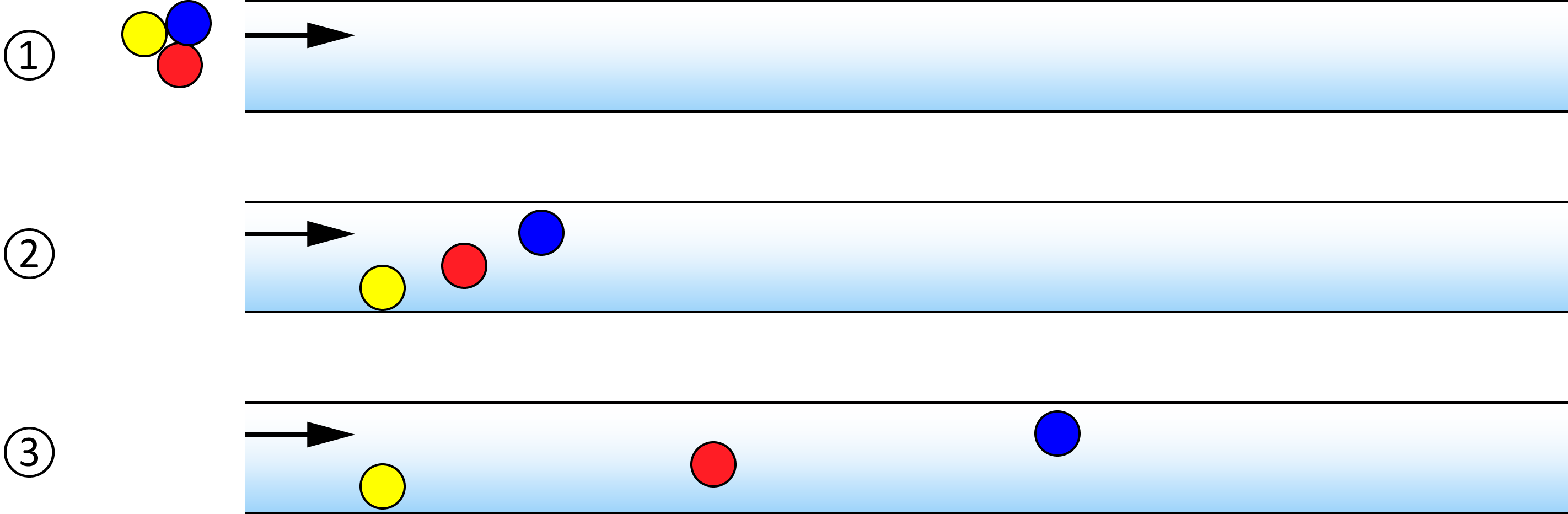
1-3-1 Separation Mechanism
This section explains the role of separation in GC. The sample mixture vaporized in the sample inlet is carried by the carrier gas and enters the column. Inside the column, each sample component interacts with (adsorbs to) the stationary phase and is selectively delayed, resulting in a distribution of arrival times at the detector. In this way, the GC column separates the components in a mixture.
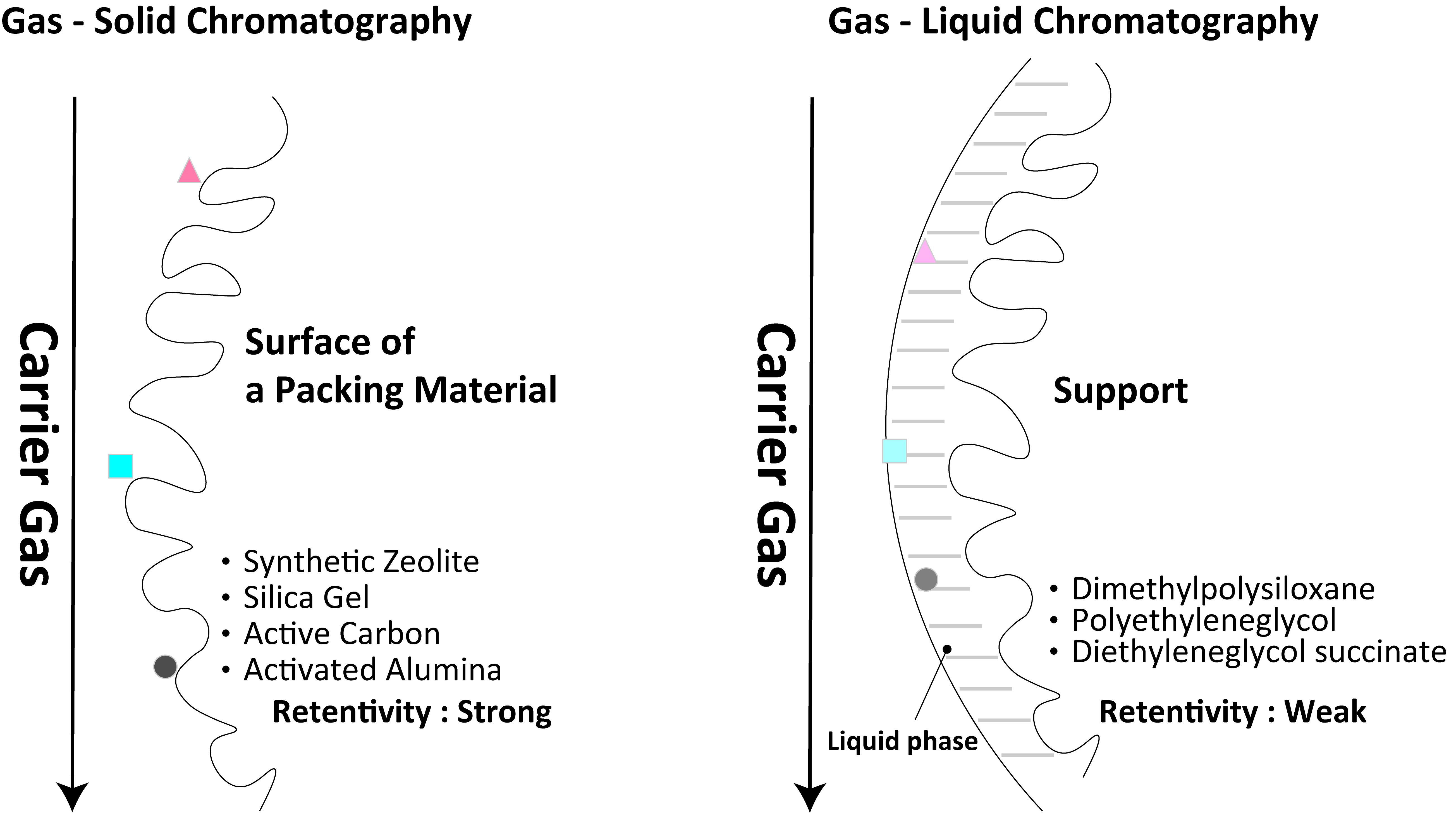
1-3-2 Classification of Stationary Phases
Stationary phases are classified into gas–solid chromatography and gas–liquid chromatography. In gas–solid chromatography, a sample is separated from the stationary phase (adsorbent) by adsorption. Rare gases and low-grade hydrocarbons can be separated on silica gel, activated carbon, alumina, and synthetic zeolite. In gas–liquid chromatography, the stationary phase is a liquid phase and the components are separated by partitioning. Common liquid phases are dimethylpolysiloxane and polyethylene glycol, which are generally used in the separation of organic compounds.
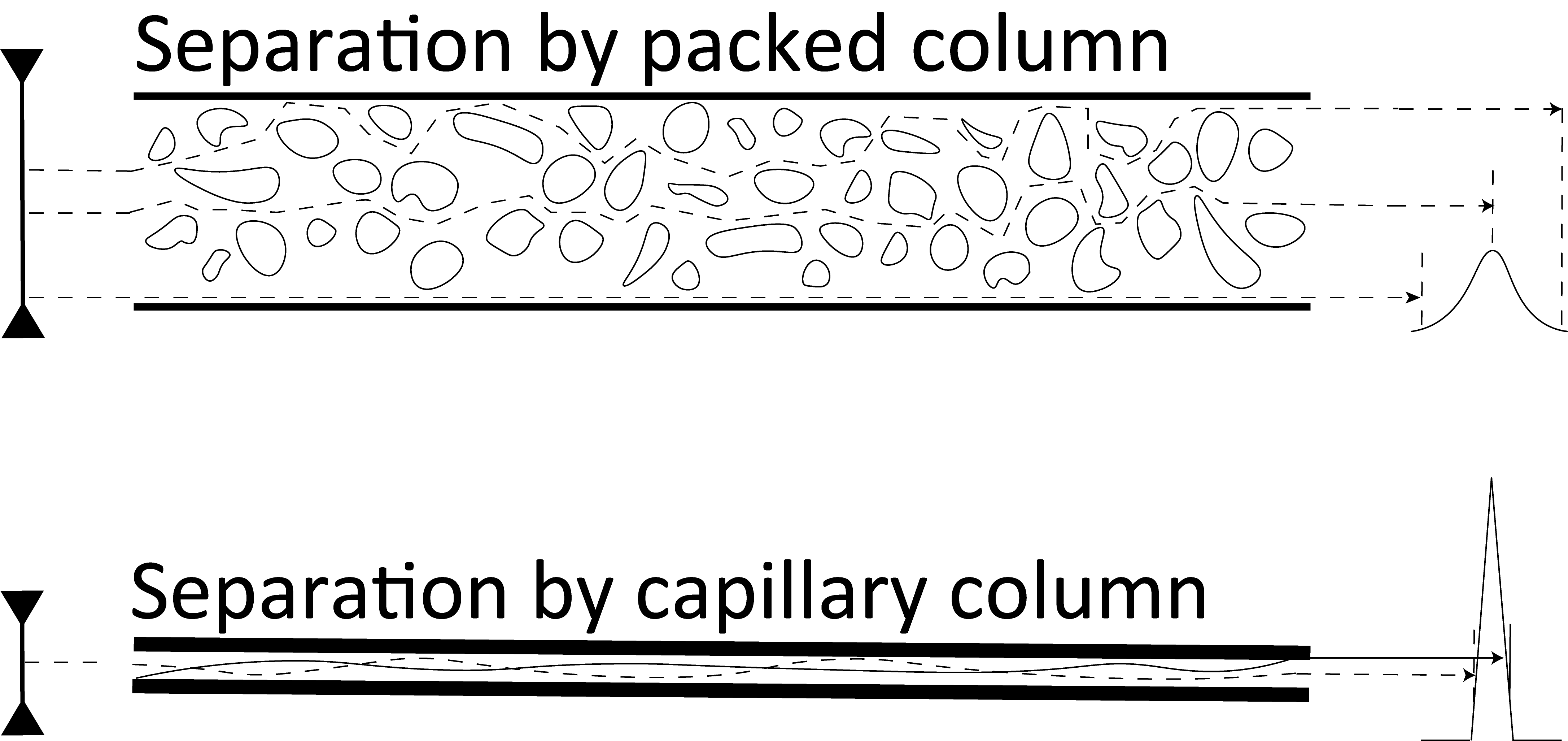
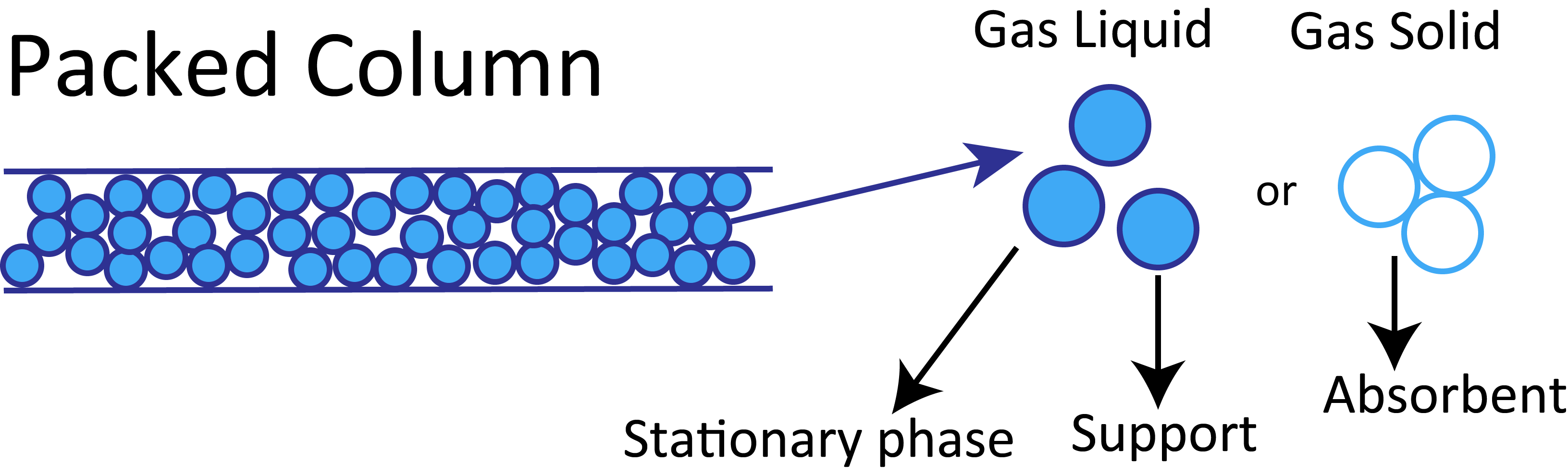
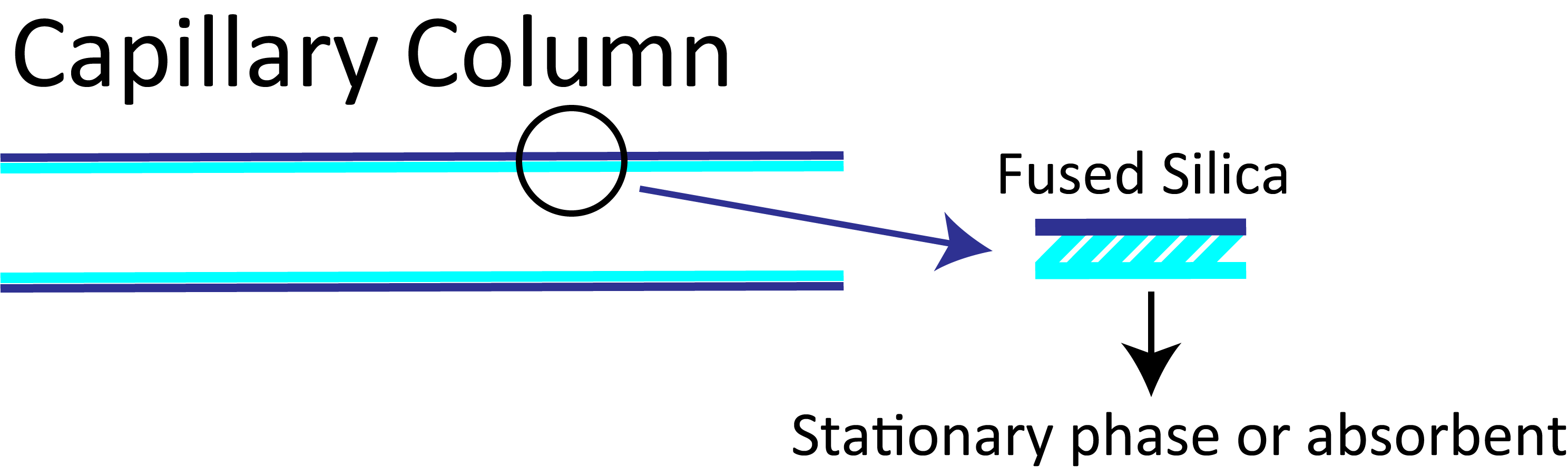
1-3-3 Classification of Columns
Columns are classified into packed and capillary columns. Packed columns are filled with a packing material in the form of particles. The typical tube of a packed column has an inner diameter of 2–4 mm and is composed of stainless steel or glass. In capillary columns, the liquid phase and packing material are held on the inner surface of a tube made of fused silica or stainless steel. The inner diameter of the tube is 1 mm or smaller.
1-3-4 Comparison of Packed and Capillary Columns
| Packed Column | Capillary Column | |
|---|---|---|
| I.D. (mm) | 2 - 4 | - 1 |
| Length (m) | 1 - 5 | 5 - 100 |
| Thickness (µm) | 3 - 10 | 0.1 - 5.0 |
| theoretical plate number, N | 2,000 - 100,000 | 7,500 - 300,000 |
| N/m | 2,000 - 2,500 | 1,500 - 5,000 |
| Material | Glass・Stainless Steel etc | Fused silica・stainless steel etc |
| Inert | Low | High |
| Sample Load (µg) | 10 - 20 | 0.05 - 3.0 |
In packed columns, the glass or stainless steel tube is packed with a carrier or sorbent impregnated or coated with a stationary phase. A capillary column is a hollow narrow tube, which is coated or chemically bonded with a stationary phase or adsorbent on its inner surface.
A column with a large number of theoretical plates, i.e., a column with a small height equivalent to theoretical plate (HETP), generally achieves good performance; that is, small peak spread within the column. Peak spreading in a column is expressed by the Van Deemter equation:
HETP = A + B / u + (Cs + Cm) u
A : Eddy Diffusion Coefficient
B : Molecular Diffusion Coefficient in The Carrier Gas
Cs : The Mass Transfer Resistances in The Stationary
Cm : The Mass Transfer Resistances in The Mobile Phases
u : The Average Linear Velocity of The Carrier Gas
In the Van Deemter equation, A is the term describing multifluid diffusion, which accounts for turbulence of the mobile phase caused by eddies in the column. However, in a capillary column, the peak broadening due to the A term is negligible because there are no multiple flow paths as in packed columns.