Objectives of Solid Phase Extraction (SPE)
How to Select a Sorbent
How to Select a Sorbent Depending on the Sample Matrix and Target Analyte
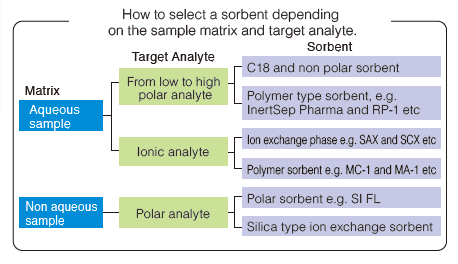
One of the most important elements to achieve successful of solid phase extraction is selection of a sorbent which should be suitable for both the sample matrix and the target analyte.
The sorbent should be carefully selected, taking into account the chemical properties of the target analyte, such as its solvent solubility and the physical nature of the sample matrix. In addition, it is important to develop conditions that are optimal for retaining the target analyte, while removing the sample matrix, then selecting an elution solvent for maximum recovery of the target analyte.
(1) Ion Exchange Phase (SAX, SCX, etc.)
(2) Specialty Phase (PBA, etc.)
(3) Mix Phase (Certify, MPC, etc.)
(4) Polar Phase (SI, FL, etc.)
(5) Non-Polar Phase (Active Carbon, SDB, C18, etc.)
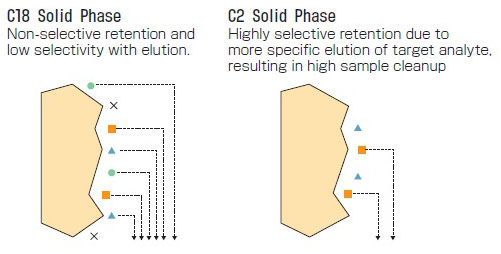
Selectivity of SPE
In order to effectively utilize the solid phase extraction method, it is important to choose a sorbent amount and size appropriate to the loading amount of the matrix and the desired product. Generally, the total retention of the cartridge type (including contaminants) is said to be up to 5 % of the solid phase loading.
In order to effectively utilize the solid phase extraction method, it is important to choose sorbent mass and size appropriate to loading amount of the matrix and the target analyte. Generally, the total retentive capacity of cartridge type (including matrix) is said to up to 5 % of the sorbent mass.
Retentive Capacity of a Sorbent Compared to Sorbent Mass

※Bed volume, this is the quantity of the solvent necessary to replace the air trapped in the solid phase. Void volume is equivalent to the bed volume
Recommendation for Selecting an Ion Exchange Sorbent
| Target Analytes |
InertSep
|
Structure |
Target Ion
|
||
|---|---|---|---|---|---|
| Weak Ion | Strong Ion | ||||
|
Acidic
|
Anion Exchange
|
MA-1 3 Class Amine
|
-CH2-N(R)3
|
✓
|
✓
|
|
MA-2 2 Class Amine
|
-CH2-N(R)2
|
|
✓
|
||
|
NH2 Aminopropyl
|
-CH2 CH2 CH2 NH2
|
|
✓
|
||
|
PSA 1 Class, 2 Class Amine
|
-CH2 CH2 CH2 NHCH2 CH2 NH2
|
|
✓
|
||
|
SAX Tri-Methylaminopropyl
|
-CH2 CH2 CH2 N+(CH3)3
|
✓
|
|
||
|
Basic
|
Cation Exchange
|
MC-1 Sulfonic Acid
|
-CH2-SO3-
|
✓
|
|
|
MC-2 Carboxylic Acid
|
-CH2-COO-
|
|
|
||
|
CBA Ethyl Carboxylic Acid
|
-CH2 CH2 COO-
|
|
✓
|
||
|
PRS Propyl Sulfonic Acid
|
-CH2 CH2 CH2 SO3-
|
✓
|
|
||
|
SCX Benzene Sulfonic Acid
|
-CH2 CH2 C6 H4 SO3-
|
✓
|
|
||


























